A stage 3 clinical trial has proven the efficacy of lacosamide alone to treat focal seizures, specifically as a first-line treatment for newly diagnosed epilepsy.
Epilepsy is one of the most common neurological illnesses, with recurring seizures that can alter information transfer by neurons. It affects roughly 430,000 individuals in France and over 50 million individuals around the world. Several treatments are available however none are currently curative. The efficacy and adverse side effects of antiepileptic drugs vary from one person to the next.
Selecting initial treatment for newly diagnosed epilepsy in adults is essential and depends on a number of parameters: type(s) of seizures and epileptic syndrome, gender, potential pregnancy, comorbidity, co-medication… These factors reduce the number of treatments adapted to a specific patient, which is why the medical community hopes to see the rise of new single-drug treatment possibilities.
In a stage 3 clinical trial, Michel Baulac and colleagues tested the efficacy of lacosamide alone as a first-line treatment in newly diagnosed adults with epilepsy. They compared it with carbamazepine, a frequently prescribed treatment with established efficacy.
Lacosamide has been prescribed as an antiepileptic drug since 2008. It was first used as additive therapy for patients with focal seizures (with or without secondary generalization). It has limited side effects and a low risk of drug interaction.
The study was conducted on 888 patients over the course of up to 2 years for some patients. Half of the patients, selected at random, were administered lacosamide and the other half were administered extended-release carbamazepine.
Proof of lacosamide efficacy relies on the observation of a percentage of patients without seizure relapses for 6 months equivalent to the percentage observed with carbamazepine. Both treatments were well tolerated, with certain differences: increased drowsiness with carbamazepine, increased dizziness with lacosamide.
These results suggest that lacosamide is a good candidate for treating epilepsy in newly diagnosed adult patients.
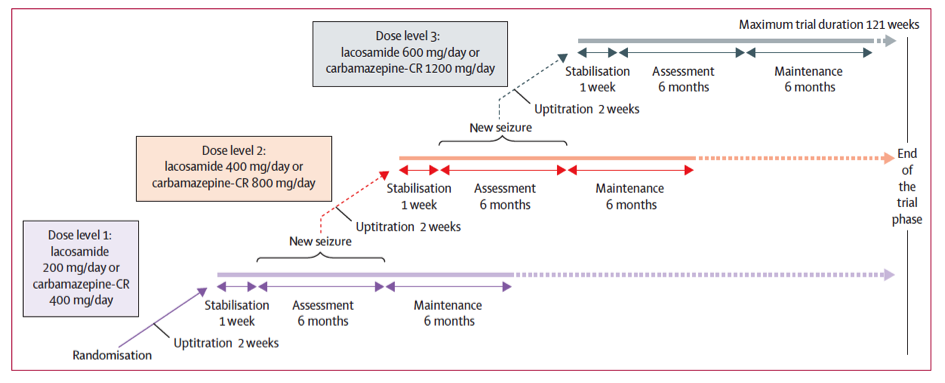
The protocol, a repetition of clinical practice patterns, was the following: after a plateau, patients were administered treatments with a 200mg daily dose for lacosamide or 400mg daily dose for carbamazepine. In case of seizures, the dosage was increased up to 600mg daily for lacosamide and 1200mg daily for carbamazepine. Patients with no seizures over the course of the 6-month treatment entered a stabilization phase with the same dosage as the previous 6 months. Treatment efficacy was therefore evaluated in patients who did not experience seizures for 6 consecutive months after stabilization with the last dosage tested.
Reference : Efficacy, safety, and tolerability of lacosamide monotherapy versus controlled-release carbamazepine in patients with newly diagnosed epilepsy: a phase 3, randomized, double-blind, non-inferiority trial. Michel Baulac, Felix Rosenow, Manuel Toledo, Kiyohito Terada, Ting Li, Marc De Backer, Konrad J Werhahn, Melissa Brock.